Before planting any plants in his garden, vegetable garden or summer cottage, the gardener must examine the condition of the soil.
It is important to know not only the composition of the soil and its fertility, but also such an important parameter as acidity. The ability of plants to assimilate nutrients from the soil depends on the value of this parameter.
In addition, soils with different acidity react completely differently to fertilization. The disturbed acidity of the soil leads to the fact that many nutrients will simply not be absorbed by plants. Moreover, the amount of these substances will not play any role.
Content:
 Read also: Projects of country houses for 6-10 acres: 120 photos, description and requirements | The most interesting ideas
Read also: Projects of country houses for 6-10 acres: 120 photos, description and requirements | The most interesting ideas
Introduction

Soil acidity - how to determine
Most agricultural crops successfully grow and bear fruit on soils of neutral acidity. Too acidic or, conversely, too alkaline soil is dangerous for plants and many beneficial bacteria and other microorganisms involved in metabolic processes and forming humus.
In fact, if the soil acidity parameter on the site differs too much from neutral values, you can forget about good harvests and regeneration of the fertility of the site.
 Read also: How to make a patio in the country with your own hands: a variety of design options, decoration and arrangement (85+ Photo Ideas & Video)
Read also: How to make a patio in the country with your own hands: a variety of design options, decoration and arrangement (85+ Photo Ideas & Video) What is soil acidity

Fertilization is one of the reasons for increasing soil acidity.
Acidity is a value that characterizes the content of hydrogen ions in the soil. It is indicated by the pH parameter, which can vary quantitatively from 0 to 14.
At the same time, depending on its size, the following types of soils are distinguished:
- very acidic (pH less than 4.0);
- strongly acidic (pH 4.1-4.5);
- medium acid (4.6-5.0);
- slightly acidic (5.1-5.5);
- neutral (5.6-8.4);
- slightly alkaline (8.5-8.9);
- medium alkaline (9.0-9.4), etc.
In the regions belonging to the CIS, Most soils are either neutral or slightly acidic.
The main problem of soils is the acidification process caused by a gradual increase in the concentration of hydrogen ions. This is a natural process, the main reason for which is the consumption of nutrients from the soil by plants. In order to somehow give plants more nutrition, gardeners and gardeners use various preparations containing the substances they need.

Ammonium nitrate
Unfortunately, for normal assimilation by plants, these substances must be in the composition of some kind of acids.
So, for example, almost all nitrates (nitrogenous fertilizers) are salts of nitric acid, potassium sulfate is a salt of sulfuric acid, etc. When mixed with water, fertilizers dissolve and partially decompose into acidic residues, which cause acidification of the soil. Thus, abundantly nourishing plantings, we ourselves create problems for ourselves that we will face in the near future.
Of course, the acidification of the garden (and even more garden) plot will not occur within one season, but if, within 3-5 years of fertilizing, their effect on the soil is not neutralized in any way, we will end up with a rather acidic soil with a low pH.
It is important that a similar result is obtained if the land is fertilized with top dressing in any form: both mineral and organiccompost, manure, peat fertilizers, etc.)
 Read also: Fertilizer for indoor plants. Description of fertilizer types, home dressing recipes (Photo & Video) + Reviews
Read also: Fertilizer for indoor plants. Description of fertilizer types, home dressing recipes (Photo & Video) + Reviews
Acidity and trace elements

Plant chlorosis, one of the causes of which is insufficient absorption of magnesium on acidic soils
Trace elements in the soil are unevenly distributed. This is due to the different chemical composition of certain areas, and also depends on the fertilizers applied to it. But this is not the main problem. Depending on the pH level, each microelement can be activated by the plant metabolism system in different ways.
Simply put, each trace element has its own acidity value at which it can be absorbed well, as well as such levels of acidity at which the element is not absorbed at all. Moreover, no matter how much it is in the soil initially, no matter how much the gardener makes in the form of dressings, at certain acidity values, this element will not be absorbed at all.
This question is very important, since it is he who shows the limitations of growing certain crops on various soils. For example, nitrogen is well assimilated on neutral soils, and with a significant deviation of acidity from the norm (up to 4.5 or up to 9), the degree of its digestibility drops by almost half.
Traditionally, there are "acidic" elements that are well absorbed on neutral and acidic soils (pH less than 7.5-8):
- iron;
- manganese;
- boron;
- copper;
- zinc.
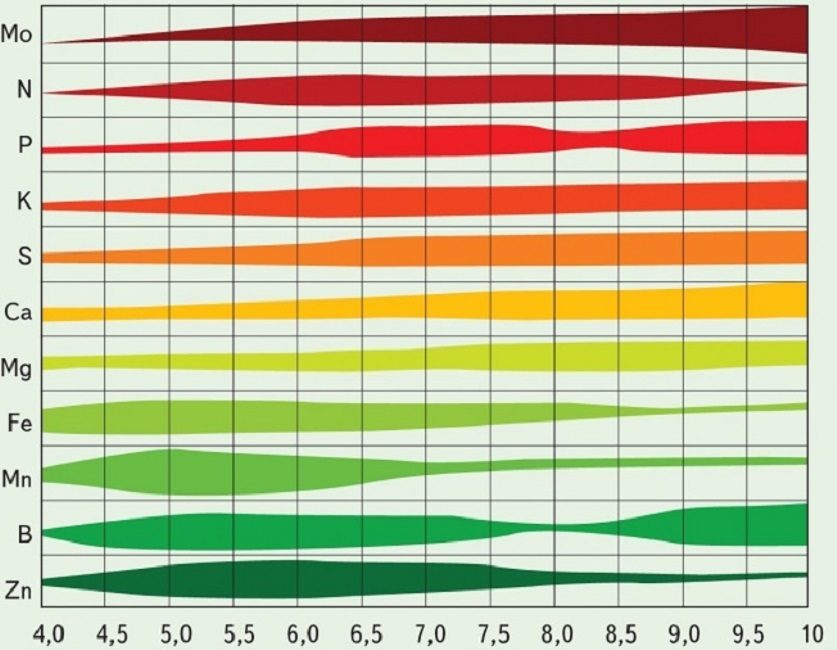
An approximate graph of the availability of mineral nutrition for plants on soils of different acidity (the greater the thickness, the better the microelement is absorbed)
As well as "alkaline" - representing, as it were, the opposite of those previously considered, well processed by plants on neutral and alkaline soils (pH more than 6-6.5):
- potassium;
- calcium;
- magnesium;
- molybdenum.
In addition, there are elements, such as nitrogen and sulfur, that have more or less the same assimilation at almost any acidity. Phosphorus stands apart, which “loves” neutral or very alkaline soil (pH above 8.5), and practically does not enter plants in strongly acidic soil.
If we analyze the presented graph, we can draw several conclusions:
- The most critical substances for plants - potassium, nitrogen, calcium and sulfur are very poorly absorbed in acidic soils (pH below 5.0-5.5). Therefore, it is strongly recommended to deoxidize too acidic soils so that these microelements applied with fertilizers are well absorbed by plants.
- There is a certain optimal acidity zone (pH from 6.0 to 7.0), in which almost all microelements enter plants equally well. Such pH values correspond to neutrally acidic soils: chernozem, dry soddy soil and heavy loam. It is on these types of soils that fertilizers give the maximum effect.

Device for determining soil acidity pH - meter
 Read also: Making a compost box with your own hands: a description of the main technical points, recipes for making compost (50 Photos & Videos) + Reviews
Read also: Making a compost box with your own hands: a description of the main technical points, recipes for making compost (50 Photos & Videos) + Reviews Determination of soil acidity

TL-H2 instrument used to determine soil acidity
There are several methods for determining soil acidity, differing in efficiency, accuracy and cost. There are even measuring devices for this purpose. However, for everyday tasks, you can use the simplest methods, since the main goal of such “measurements” will be to answer the question: do you need to deoxidize the site or does the gardener still have some time to spare.
The easiest way to determine soil acidity is to use litmus paper. It changes its color depending on the pH value, that is, it is an indicator of acidity. This cheap remedy can be purchased at any pharmacy or hardware store.
The measurement process itself is quite simple: you should take soil samples, wrap them in a dense cloth and fill them with distilled water in a ratio of 1 to 1. After about 5-7 minutes, you need to lower the litmus paper into a container of water for a few seconds. The color of the indicator paper is compared with the acidity scale and pH is determined from it.

A package of litmus paper with an acidity scale printed on it
There is also a simple, albeit very approximate, way to determine acidity at home using vinegar and soda. Soil samples are placed in two flat containers and filled with water in a ratio of 2 to 1. Next, add vinegar to the first container, and add a soda solution to the second. If the release of gases begins in the first container, the soil is alkaline, in the second - acidic. If the water does not foam in any of the containers, the soil is considered neutral.

Determination of soil acidity using vinegar and soda
Another way to roughly estimate the acidity of the soil is by looking at the weeds growing on it. As a rule, horsetail, sorrel, plantain, tricolor violet grow on acidic soils.
Depending on which cultivated plants are grown in certain parts of the garden, the assessment of normal acidity may be different. The optimal acidity for some crops is shown below:
| culture | Optimal acidity value |
|---|---|
fruit plants | |
| Cherry, plum | 7.0 |
Apple trees, pears, gooseberries, currants | 6.0 – 6.5 |
Nut | 6.5 – 7.5 |
Apricot | 6.0 – 7.5 |
Quince | 5.0 – 6.5 |
Garden and vegetable crops | |
tomatoes | 6.0 – 6.5 |
cucumbers | 7.0 |
Carrot | 5.6 – 7.0 |
Beet | 6.2 – 7.0 |
Sorrel | 4.1 – 5.0 |
Potato | 5.2 – 5.7 |
radish | 5.5 – 6.0 |
Pumpkin | 6.5 – 7.0 |
Peas, Legumes | 6.0 – 6.5 |
Cabbage | 6.2 – 7.5 |
Berry crops | |
Blackberry | 6.0 – 6.6 |
Strawberry wild-strawberry | 5.0 – 5.5 |
Raspberries | 5.5 – 6.0 |
Plants that prefer to grow in acidic soils | |
Rhododendron | 4.0 – 5.5 |
Hydrangea | 2.0 – 4.5 |
Cowberry | 3.0 – 5.0 |
Cranberry | 3.5 – 5.2 |
Fern | 4.5 – 6.0 |
Blueberry | 3.5 – 4.5 |
Heather | 3.5 – 4.5 |
It is necessary to know the pH value for all crops growing on the site, so that in case of too low values of this parameter (corresponding to increased acidity) take measures to neutralize the acidic environment.
![[Instructions] How to make beautiful and unusual wall shelves with your own hands: for flowers, books, TV, kitchen or garage (100+ Photo Ideas & Videos) + Reviews](https://iherb.bedbugus.biz/wp-content/uploads/2018/05/19-6-300x213.jpg) Read also: [Instructions] How to make beautiful and unusual wall shelves with your own hands: for flowers, books, TV, kitchen or garage (100+ Photo Ideas & Videos) + Reviews
Read also: [Instructions] How to make beautiful and unusual wall shelves with your own hands: for flowers, books, TV, kitchen or garage (100+ Photo Ideas & Videos) + Reviews
Soil deoxidation methods

Soil deoxidation
Soil deacidification (often referred to as liming) is currently the only agricultural practice to reduce soil acidity. Its essence lies in the addition of calcium compounds to the soil. Preferably, this will be hydroxide (or lime) or carbonate (or chalk). In some cases, other components are also used.
The choice of calcium is due to its minimal negative impact on the soil. Moreover, for the normal development of most plants, calcium in one form or another is necessary.
Thus, soil deoxidation combines two beneficial actions in one: reduces the acidity of the soil and enriches it with a valuable trace element. Below are the various methods of soil deoxidation
Lime

Applying lime to acidic soil
Sometimes it is replaced by calcareous tuff, ground limestone (limestone flour), cement dust, marsh drywall, etc. All these substances have the same principle of action, only their application rates differ.
Usually, fluff is brought in in the fall, so that during the winter period all chemical processes are completely completed. Application rates are shown in the table below:
| Soil acidity | Lime application rate |
|---|---|
| 4.1 – 4.5 | 500 g per 1 sq. m. |
4.6 – 5.0 | 300 g per 1 sq. m. |
5.1 – 5.5 | 200 g per 1 sq. m. |
These standards do not depend on soil types. If ground limestone is used instead of lime, the type of soil plays a role. On heavy soils, dosages will generally be higher.
| Soil acidity | Application rate on sandy loam and light loam | On heavy loams |
|---|---|---|
| 4.1 – 4.5 | 400 g per 1 square. m. | 600 g per 1 sq. m |
4.6 – 5.0 | 300 g per 1 sq. m. | 500 g per 1 sq. m |
5.1 – 5.5 | 200 g per 1 sq. m. | 400 g per 1 square. m |
The application procedure itself is very simple: lime is evenly scattered over the surface, adhering to the specified norms, and then they dig the site to a depth of at least 20 cm.
Dolomite flour

Application of dolomite flour under fruit trees
Dolomite flour is a crushed dolomite (it is a mineral, consisting of a complex compound of calcium and magnesium carbonates). It is much more convenient to use, since it is not as dangerous to humans as lime, in addition, it can be applied both in autumn and in spring.
Another important property of dolomite flour is its ability to loosen too heavy and viscous clay soils. This improves not only their mineral composition, but also the degree of friability, thereby increasing the efficiency of plant root respiration.
The amount of dolomite flour introduced, depending on the acidity, is given in the table:
| Soil acidity | Application rate of crushed dolomite |
|---|---|
| 4.1 – 4.5 | 500 g per 1 sq. m. |
4.6 – 5.0 | 400 g per 1 square. m. |
5.1 – 5.5 | 300-400 g per 1 sq. m. |
The introduction is similar to lime - uniform distribution of the drug in the consistency of the powder over the area, followed by digging to a depth of 20-30 cm.
Ash

A bucket of wood ash is enough to deoxidize 20-25 square meters. m of soil
Material that is always at hand. You can get this folk remedy on your own: just burn the cut branches of trees, dead wood, etc. It is not only a deoxidizer, but also an excellent complex fertilizer rich in trace elements.
But it should be remembered that ash has some drawbacks. The first is quantitative: given the low density of this substance, it is rather problematic to obtain it in large quantities. The second is qualitative: depending on the wood used for burning, the amount of calcium compounds in the ash can be from 1/3 to 2/3, that is, the application rates can vary significantly.
For strongly and moderately acidic soils, application rates of the order of 1.0-1.5 kg of ash per 1 square meter are used. m, if tree ash with thick wood is used. To an inexperienced gardener, this norm will seem insignificant, but in fact it is a very large amount of ash, since one glass of it weighs about 100 grams.
When grass and weeds are burned instead of wood, the norms are increased several times (up to 2.5-3 kg per 1 sq. m).
This is too much ash. To get 1 kg of product, you need to burn at least 7-10 kg of waste, which can be problematic. Therefore, ash should be used not as a deoxidizer, but as a complex fertilizer.
chalk

Large chalk crystals require grinding before application
This is a more "sparing" material than lime, since it does not have such a high chemical activity. The degree of dissolution of chalk in water is very weak, therefore, before starting the liming procedure, it must be crushed very carefully. Chalk should be in the form of a fine powder without large lumps.
Application rates for loamy and clay soils range from 200 to 600 g per 1 sq. m. For sandy or sandy soils, it is added at the rate of 100-200 g per 1 sq. m. Reapplying chalk should be carried out after 2-3 years.
When making chalk, you should dig the soil to a depth of 20-25 cm.
It is best to deoxidize the beds with chalk in the spring, because when it is laid before winter, it will be washed with melt water.
Soda

Soda during deoxidation is introduced in the form of a solution in water
Baking soda or sodium bicarbonate can also be used to deacidify the soil. The advantages of this method to reduce acidity include its almost instantaneous effect on the soil. The disadvantage is the presence of sodium in it. This element tends to accumulate in the soil and inhibit the growth of plants, especially young or seedlings. Therefore, soda is used in small quantities, and mainly in the form of a solution.
To deoxidize the site, use a solution of 100 g of soda in 1 liter of water. This amount is enough to process 1 square. m of soil. It is advisable to evenly spray the solution with a spray gun, and then carefully treat the area with a rake.
Liming the soil with soda in greenhouses is also undesirable. Even if you apply it a little, it will negatively affect young plants.
Soda is used to deoxidize only the topsoil, since a larger amount of the substance has a negative effect on the soil.
Gypsum

Gypsum
Material similar in characteristics to chalk, but it has the advantage of reacting with acid much more quickly, without having any negative effect on plants or humans.
In addition, excess gypsum is stored in the soil and reacts with acid as it occurs in the soil. Roughly speaking, gypsum is activated in the soil layer as soon as it becomes acidic again.
The application mechanism is similar to lime, dolomite flour or chalk: the top layer of soil is sprinkled with high-quality crushed gypsum and the soil is dug up to a depth of 20-30 cm.
Gypsum application rates are given in the table:
| Soil acidity | Application rate of gypsum |
|---|---|
| 4.1 – 4.5 | 400 g per 1 square. m. |
4.6 – 5.0 | 300 g per 1 sq. m. |
5.1 – 5.5 | 100-200 g per 1 sq. m. |
siderates

Rye is one of the most popular green manure
Soil quality can also be improved by other, non-chemical methods. There are several crops that require highly acidic soil to thrive. In the course of their development, they naturally reduce the concentration of hydrogen ions and acidic residues.
These plants include:
- phacelia;
- alfalfa;
- rye;
- sweet clover;
- lupine;
- mustard.
Usually they are planted at the beginning of the season. (in some cases, in the middle), and after the end of the active flowering phase, mowed, crushed and mixed with the topsoil. The decrease in pH with this method is from 0.5 to 1 unit.
Thematic video: HOW TO DEOXIDE THE SOIL AND FERTILIZE AT THE SAME TIME
HOW TO DEOXIDE THE SOIL AND FERTILIZE AT THE SAME TIME
How to deoxidize the soil in the garden? | Determination of acidity + TOP-7 Ways | (Photo & Video) +Reviews







